Colossal BioSciences made headlines around the world this month with a breakthrough that is as fascinating as it is adorable: the creation of a ‘woolly mouse’ with mammoth traits. These long-haired rodents aren’t just irresistibly cute, they represent a historic leap forward in gene editing and a major milestone on the path to bringing back the extinct woolly mammoth.
But as I discovered in my conversation with Ben Lamm, Co-Founder and CEO of Colossal, this story goes far beyond resurrecting an Ice Age giant. The company is also on the verge of reviving the thylacine—better known as the Tasmanian tiger—and with 50% of all biodiversity set to go extinct in the next 25 years, its cutting-edge technology is poised to play a critical role in addressing one of the greatest challenges of our time.
It’s bold, controversial and undeniably visionary. Here’s my conversation with Ben Lamm.
Cassandra Tanti: Colossal is working to bring back the extinct woolly mammoth, which roamed the Northern Hemisphere thousands of years ago during the last Ice Age. However, before getting there, your company has created something decidedly less mammoth—a woolly mouse. Tell us what Colossal has managed to achieve in this exciting area of de-extinction.
Ben Lamm: A lot of people got excited about the woolly mouse because they thought it was adorable—it had maximum “cute factor”—but it’s actually also a scientific marvel.
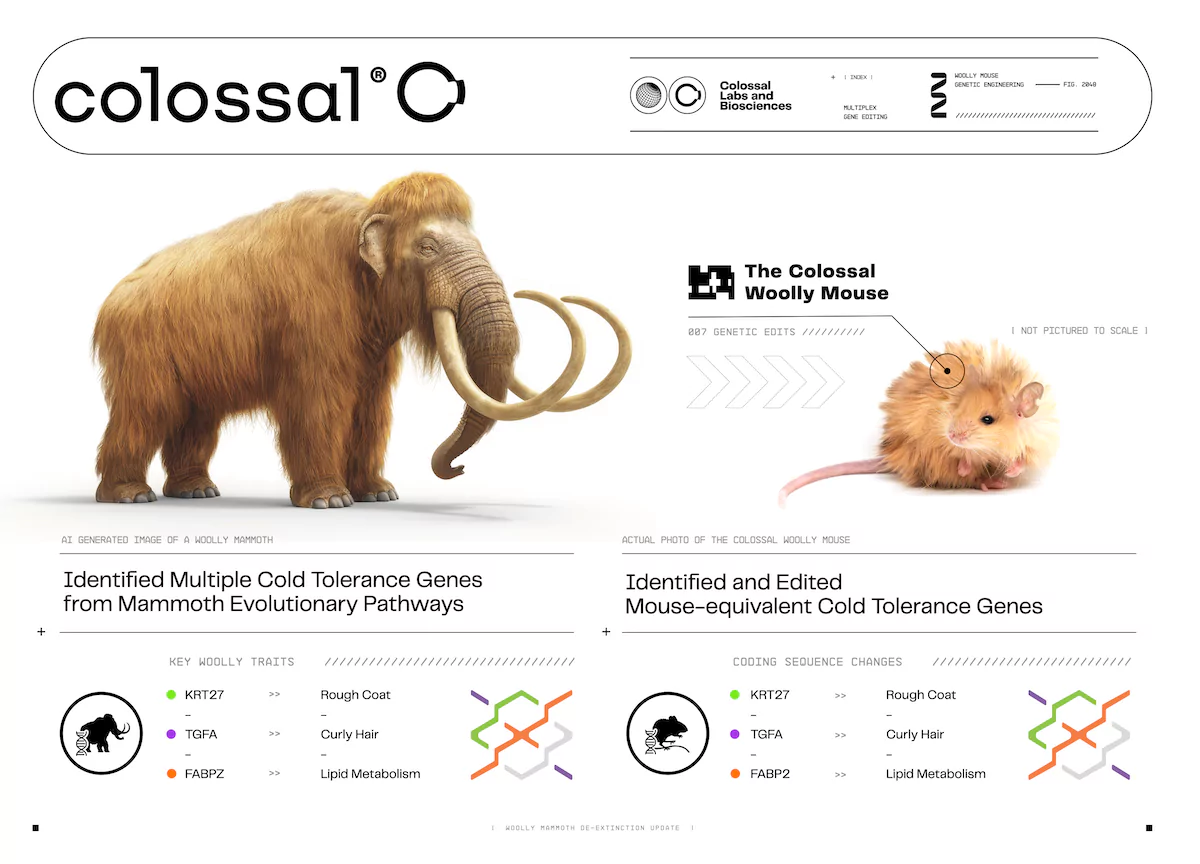
We’ve been working on bringing back the woolly mammoth for three years now. In that process, we’ve done a lot of ancient DNA work and genetic assembly, and we’re currently in the editing phase. We’re editing Asian elephant cells with genetic targets from the mammoth.

But the challenge is that there are really only three ways to test if these genetic edits work. One option is to create a mammoth—but that doesn’t seem like the best ethical approach just for testing. The second way involves creating elephant stem cells and differentiating them into various tissue types, including hair follicles, to see if we can grow mammoth hair in a lab. That would confirm that our edits are successful, but it still wouldn’t result in a full animal.
So, we decided to take a different approach. Could we map these same traits and genes—or their equivalents—in mice and engineer them accordingly? Mice have a 20-day gestation period, compared to 22 months in elephants, which allows us to see results much faster. What we found was remarkable—not only were all the traits we engineered into the Asian elephant cells working correctly, but our entire end-to-end process was validated.
We took ancient DNA, engineered it into cells, conducted cloning, performed microinjections into embryos and screened for healthy animals—and it worked immediately. We started the project in September, and the woolly mice that became an internet sensation were born in October.
That’s incredible. But where exactly did the woolly mammoth DNA come from?
We have about 59 mammoth genomes, ranging from approximately 4,500 years old to 1.2 million years old. Some of the oldest ones are over a million years old, which is amazing.
Much of this DNA comes from our academic partners, like Love Dalén, who is arguably the world’s leading mammoth researcher at Stockholm University. He and other members of the Explorers Club, which I’m also a part of, have gone into the permafrost to find frozen mammoth bones and tissues. Over the last 15 years, they’ve brought these samples back to labs for genetic sequencing.
Even though Colossal is only a few years old, the research behind this has been going on for nearly two decades.

That explains how you’re doing this, but why?
There are a few key reasons. First, conservation is severely underfunded and underdeveloped in terms of technology. It is estimated that we could lose up to 50% of all biodiversity by 2050, which is a terrifying statistic. We need new tools to combat biodiversity loss. Our perspective is that it’s better to have a de-extinction toolkit and not need it than to need it and not have it. These technologies can help bring back extinct species and also save critically endangered ones.
Second, when you tackle these problems through a systems approach, you drive innovation across multiple fields—computational biology, genetic engineering, advanced embryology and even artificial wombs. While these have applications in conservation and de-extinction, they also hold massive potential for human healthcare.
And third, kids love what we’re doing. Every week, we receive 10 to 100 letters and drawings from children who are inspired by our work. We see Colossal as a company that can excite and educate the next generation while creating real impact in conservation and biotechnology.
Since the woolly mice are only a few months old, will you be closely monitoring them—particularly to study how well they adapt to cold environments?
Exactly. It’s not just about hair—it’s about true cold adaptability. We’re studying them for six to 12 months to evaluate diet, cold exposure and temperature resilience, comparing them to wild-type mice. Everything we do goes through our ethics board, and we try to be as transparent and collaborative as possible.
And yes, that cold tolerance is a core capability for us. We’ll be publishing a paper specifically on that soon.
One of the other extinct species you’re working on is the Tasmanian tiger. As an Australian who grew up in Tasmania, I find that especially exciting. Do you have a personal motivation for bringing back something that has been lost forever?
I didn’t grow up in Australia, but I’ve spent a lot of time there and I love the thylacine—better known globally as the Tasmanian tiger. It’s one of the coolest animals. For those who don’t know, it looks like a mix between a wolf, a zebra and a kangaroo.
Unfortunately, humans hunted it to extinction. The good news is that we have incredible DNA samples. We’re working closely with the University of Melbourne, and some of the scientists there—like Dr. Andrew Pask—are among the best in the world.
I think we’ll see a Tasmanian tiger again within the next eight years. Last year, we completed 99.9% of the thylacine genome, with only a few gaps in areas that aren’t critical to our work. We’re now in the editing phase, modifying the genome of the fat-tailed dunnart, the closest living relative of the thylacine.
I love the idea that we can bring back animals that aren’t too far removed from our time. Just a few generations ago, Australians lived alongside the thylacine—it wasn’t some distant, prehistoric creature. Undoing some of the environmental damage caused by humans is a huge motivator for us.

Skepticism is important, and we welcome it. We’ve assembled a team of 170 scientists, collaborate with 17 top universities and work with Nobel laureates, conservationists and rewilding experts.
When Colossal launched, one of our biggest critics, Love Dalén, saw the scientific merit and joined us. The same happened with Dr. Beth Shapiro, another key figure in ancient DNA research.
We take ecological concerns seriously. For the Tasmanian tiger project, we’ve formed a working group that includes Indigenous communities, ecologists, conservationists, university researchers and even logging industry reps, since forestry is a major economic driver in Tasmania. We meet quarterly and run rigorous ecological field studies.
A lot of concerns are valid, and we’re working on solutions. Some people will always dislike what we’re doing—and that’s okay. But if we can inspire kids, help conservation and get 90% of people excited, we’re on the right path.
Colossal has spent a lot of time on this. If you visit colossal.com/tasmania, you’ll see that even without a living Tasmanian tiger, we’ve already built an inclusive, science-based working group. We’re also preparing peer-reviewed papers on rewilding, diet and microbiomes.
So when people raise concerns—often after seeing just a short headline or clip—I always say: reach out. We’re here to educate, not persuade. And all the technology we develop—whether for the Tasmanian tiger, the dodo or the mammoth—is shared freely with our conservation partners.
Your woolly mouse paper is unpublished at this stage. Do you think that’s partly why there’s been more skepticism? Would it be different if it were already peer-reviewed?
That’s a common misconception. Yes, we have peer-reviewed papers published, but Colossal isn’t focused on publishing for its own sake—we’re focused on creating mammoths and bringing back the Tasmanian tiger.
You can stack up hundreds of papers, but that alone won’t bring back an extinct species. Academia often tackles narrow problems, which are valuable, but they don’t always lead to system-level solutions for urgent issues like biodiversity loss.
We recently released a preprint that went viral, and from our latest Tasmanian tiger update alone, there are over 15 papers now in progress. These things take time—the peer-review process is slow.
That’s why Colossal focuses on publishing around core topics, and we let our academic partners—like Dr. Andrew Pask’s team at the University of Melbourne—handle the rest. His lab alone has more than 15 thylacine-related papers underway. Some may be out this year, others later. That’s the pace of science.

What about timelines? How close are you to bringing back the Tasmanian tiger compared to the woolly mammoth?
When we launched the Tasmanian tiger project, we estimated a 10-year timeline—and so far, we’re right on track. We’ve completed the comparative genome work, built the full genomes of related species, identified all our targets and made over 300 edits. At this pace, a living thylacine within six to eight years is realistic.
The mammoth project is also in the editing phase, though slightly simpler. Out of 85 gene targets, we’ve already edited 25 with 100% efficiency—an exceptional result in genome engineering. We’re aiming for our first woolly mammoth embryos by the end of 2026, with calves potentially arriving by late 2028.
Will that be an elephant-mammoth hybrid or a true mammoth?
In biology, hybrid has a specific meaning. Most people don’t realise it’s not as simple as mixing two species. Think of having Neanderthal DNA in your 23andMe results—it doesn’t make you a Neanderthal. You’re still Homo sapiens.
The truth is, cloning an extinct species 100% isn’t possible—we’ll never have the full genome. What we’re doing is functional de-extinction: restoring lost traits like cold tolerance, thick fur, specialised hemoglobin and other key adaptations.
It’s not about creating a perfect replica—it’s about bringing back functionality that matters for ecosystems.
And how can that same technology be used to preserve existing endangered species?
That’s a major focus for us. There’s simply not enough investment, innovation or biobanking in conservation today.
That’s why we created the Colossal Foundation—not just to donate our tech, but to raise $50 million to accelerate efforts in biobanking, new technology development and what we call “bio vaults”.
Unlike the seed vaults in Europe, there’s no global system to preserve animal biodiversity. We need to bank cell lines, stem cells, embryos, sperm, eggs and sequence genomes across entire species and individuals within species—not just once, but broadly and at scale.
This isn’t happening fast enough. One of my personal goals is to help build a global network of bio vaults—because we’re losing species faster than we can save them, and we need backup systems now.
What kind of regulatory controls are you subject to in your work?
Every region is different. The US, Europe, Australia, Mauritius—they all have different views on GMOs. But we’re not making products for human consumption like GMO corn. We’re working to rewild animals, which involves a wide array of stakeholders.
That’s why we’ve partnered with everyone—from governments and lawmakers to Indigenous communities and private landowners. This can’t be done by Colossal alone. It requires collaboration at every level.
Monaco Life is produced by real multi-media journalists writing original content. See more in our free newsletter, follow our Podcasts on Spotify, and check us out on Threads, Facebook, Instagram, LinkedIn and Tik Tok.
Main photo of Ben Lamm, Co-Founder and CEO Colossal BioSciences, provided